Kudos to Indrajit and the team for our new article in Nature Communications describing “The 20S as a stand-alone proteasome in cells can degrade the ubiquitin-tag”
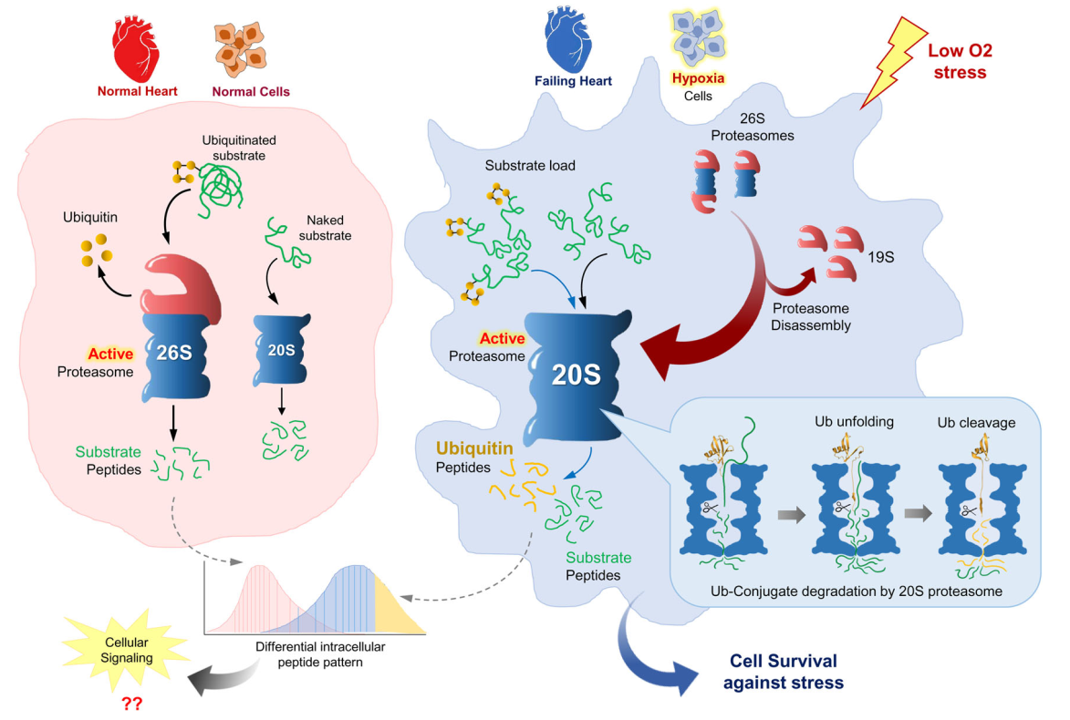
A model demonstrating the putative contribution of 20S proteasomes to proteolysis during hypoxia.
Under normoxia, the 26S proteasome degrades proteins and recycles the conjugated ubiquitin tag. Disassembly of 26S proteasomes under hypoxia leads to elevated levels of free 20S core particles (CP). Excess-free 20S CP are proposed to serve as active proteasomes capable of degrading a portion of ubiquitin along with the conjugated substrate. The disordered segment of the substrate inserts into the gate at the center of the 20S α-ring and accesses the proteolytic active sites until the ubiquitin domain is localized to the pore. Stepwise protease-driven unfolding of ubiquitin brings the conjugation region to the vicinity of the β-proteolytic active sites. Ubiquitin is proteolyzed and peptide products from both substrate and ubiquitin are released, occasionally including a remnant of ubiquitin still linked via an isopeptide bond to a substrate-derived peptide.