
Post-Doctoral fellow (TICC Post-Doc Fellowship)
Technion Integrated Cancer Centre, The Steven & Beverly Rubenstein Charitable Foundation – Fellowship Fund for Cancer Research.
http://ticc.technion.ac.il/collaboration/post-doctoral-fellowships-in-cancer-research/
Prasad Sulkshane obtained his bachelor’s degree (B.Sc. in Biotechnology) from KTHM College (University of Pune), Nashik, Maharashtra, India in 2007 and then completed Masters (M.Sc. in Biotechnology) from Deogiri College (Dr. BAM University), Aurangabad, Maharashtra, India in 2009. Prasad then moved to Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre in Kharghar, Navi Mumbai, Maharashtra, India in 2010 and obtained his Ph.D. degree in Life Sciences (from HBNI University, Mumbai) in 2016. He next joined Glickman laboratory at the Technion as a Post-Doctoral fellow in June 2017.
E mail: prasads@technion.ac.il
https://www.researchgate.net/profile/Prasad_Sulkshane
Project title: “Investigating the role of ubiquitin-Proteasomal System in the maintenance of mitochondrial homeostasis in response to hypoxia”
Tumor hypoxia favors the selection of more malignant and aggressive tumor cell phenotypes, often increasing resistance to anticancer therapeutics. Excessive and unregulated generation of Reactive oxygen species (ROS) at the electron transport chain (ETC) in response to hypoxia may cause oxidative damage nonspecifically to various cellular components and subcellular organelles including mitochondria. Recent evidence suggests that the cytosolic Ubiquitin-Proteasomal System (UPS) plays an important role in regulating the composition of mitochondrial proteome and dynamics. Ubiquitination at the mitochondria may either lead to the selective degradation of individual mitochondrial proteins by the proteasome through the Mitochondria-associated degradation (MAD) pathway or elimination of damaged/dysfunctional mitochondria through selective autophagy (Mitophagy). Hypoxia has been shown to primarily induce ubiquitination-independent mitophagy. However, our preliminary studies demonstrate persuasive ubiquitination at the mitochondria in response to hypoxia mimicking conditions, which appears to be upstream of mitochondrial fragmentation. The role of UPS in determining the effect of hypoxia on mitochondrial fate has thus been poorly understood.
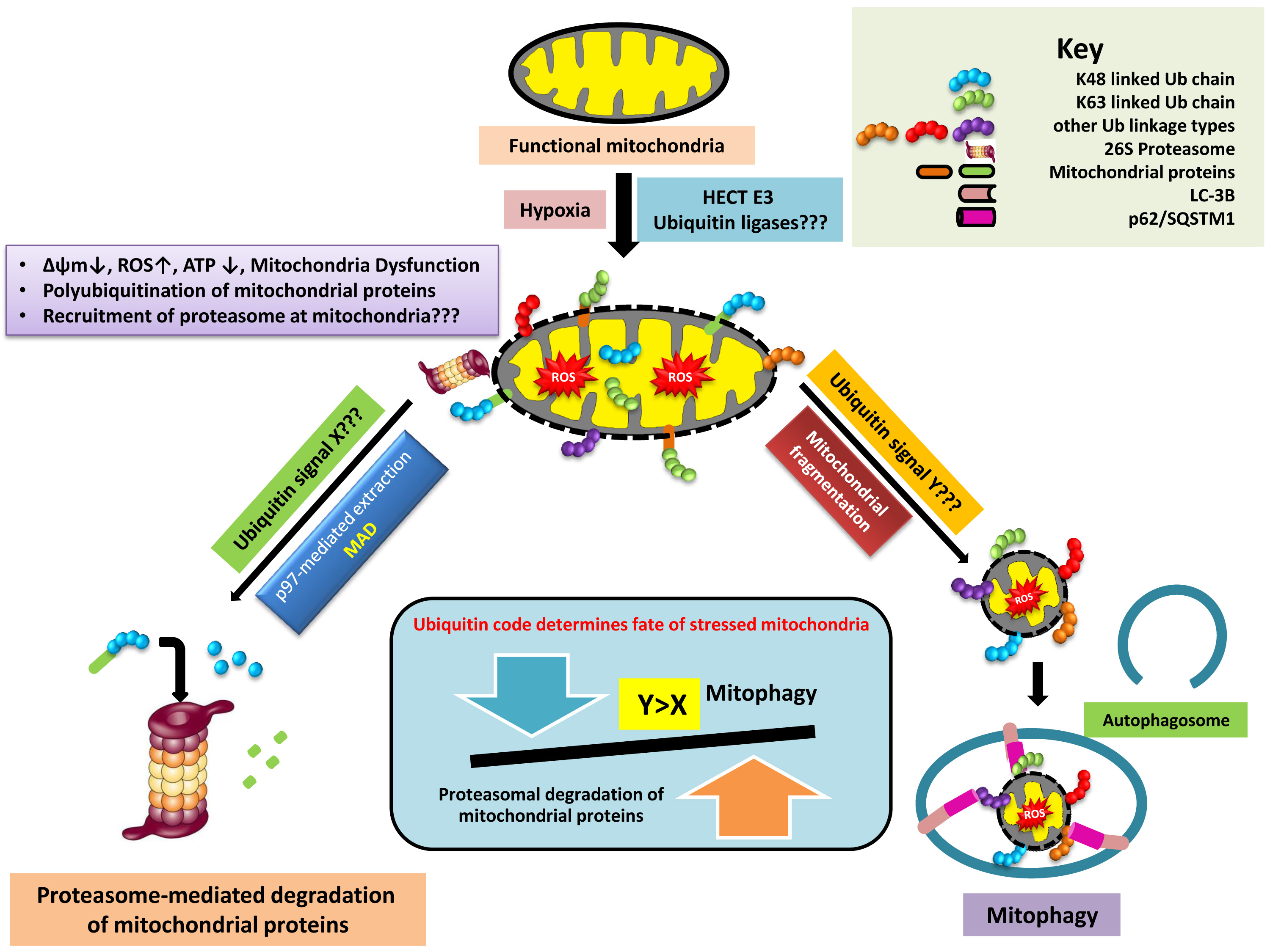
In the present research proposal, we aim to investigate the contribution of UPS in modulating the effect of hypoxia on mitochondria. We seek to map the changes in mitochondrial ubiquitinome in response to hypoxia and identify the responsible E3 ubiquitin ligases. We also aim to decipher the hypoxia-induced ubiquitin code at the mitochondria, which we believe determines the partitioning of mitochondria into either MAD-mediated degradation or Mitophagy. By employing a high throughput screen, we intend to identify UPS components important for hypoxia-induced mitophagy. We extend our research to evaluating the contribution of the proteasome in hypoxia-induced mitophagy to determine whether it serves in a parallel alternative manner to mitophagy or in a sequential manner preceding mitophagy by generating a trigger. Further, we seek to identify the components of the UPS critical in regulating the Mitochondrial dynamics (Fusion and Fission events) which aid in maintaining a healthy pool of mitochondria upon exposure to hypoxia. Answers to these questions will provide insights into how UPS may modulate mitochondrial physiology in response to hypoxia and enable tumor cell viability under conditions of oxygen deprivation. It may also be instrumental in studying mitochondria dysfunction-associated disorders in humans.
Publication
- Sulkshane P, Ram J,Thakur A,Reis N, Kleifeld O, Glickman MH. Ubiquitination and receptor-mediated mitophagy converge to eliminate oxidation-damaged mitochondria during hypoxia. Redox Biology. 2021 June 17: https://doi.org/10.1016/j.redox.2021.102047
- Sulkshane P, Pawar SN, Waghole R, Pawar SS, Rajput P, Uthale A, Oak S, Kalkar P, Wani H, Patil R, Nair S, Rane P, Teni T. Elevated USP9X drives early-to-late-stage oral tumorigenesis via stabilisation of anti-apoptotic MCL-1 protein and impacts outcome in oral cancers.Br J Cancer. 2021 Jun 2. doi: 10.1038/s41416-021-01421-x.
- Vamisetti GB, Satish G, Sulkshane P, Mann G, Glickman MH, Brik A. On-Demand Detachment of Succinimides on Cysteine to Facilitate (Semi)Synthesis of Challenging Proteins. J Am Chem Soc. 2020 Nov 18;142(46):19558-19569. doi: 10.1021/jacs.0c07663. Epub 2020 Nov 2.
- Sulkshane P, Ram J, Glickman MH. Ubiquitination of Intramitochondrial Proteins: Implications for Metabolic Adaptability. Biomolecules. 2020 Nov 16;10(11):1559. doi: 10.3390/biom10111559 [Review]
- Sulkshane P, Duek I, Ram J, Thakur A, Reis N, Ziv T, Glickman MH. Inhibition of proteasome reveals basal mitochondrial ubiquitination. J Proteomics. 2020 Oct 30;229:103949. doi: 10.1016/j.jprot.2020.103949.
- Shah D, Sulkshane P, Lalwani R, Pawar S, Teni T, Kakade A. Comparative evaluation of the clonogenic capacity of periodontal ligament fibroblasts in Hank’s balanced salt solution and egg albumen: An in vitro study. Dent Traumatol. 2018 May 4. doi: 10.1111/edt.12408.
- Sulkshane P, Teni T. BH3 mimetic Obatoclax (GX15-070) mediates mitochondrial stress predominantly via MCL-1 inhibition and induces autophagy-dependent necroptosis in human oral cancer cells. Oncotarget, 2016 August (DOI: 10.18632/oncotarget.11085)
